
Substitution preferences:
All protein types:
| Favoured | ||||||||
| Neutral | Ala ( 0) | Asn ( 0) | Ser ( 0) | |||||
| Disfavoured | Asp (-1) | Glu (-2) | His (-2) | Lys (-2) | Pro (-2) | Gln (-2) | Arg (-2) | Thr (-2) |
| Trp (-2) | Cys (-3) | Met (-3) | Phe (-3) | Tyr (-3) | Val (-3) | Ile (-4) | Leu (-4) | |
| Favoured | ||||||||
| Neutral | Ala ( 0) | Arg ( 0) | Asp ( 0) | Ser ( 0) | Lys ( 0) | Pro ( 0) | Asn ( 0) | Gln ( 0) |
| Disfavoured | Cys (-1) | Glu (-1) | His (-1) | Thr (-1) | Val (-2) | Trp (-2) | Tyr (-2) | Met (-2) |
| Leu (-3) | Phe (-3) | Ile (-3) |
| Favoured | ||||||||
| Neutral | Ala ( 0) | Gln ( 0) | Pro ( 0) | Asp ( 0) | Thr ( 0) | Asn ( 0) | His ( 0) | Arg ( 0) |
| Ser ( 0) | ||||||||
| Disfavoured | Glu (-1) | Lys (-1) | Met (-2) | Leu (-2) | Val (-2) | Trp (-2) | Ile (-2) | Tyr (-2) |
| Phe (-3) | Cys (-6) |
| Favoured | Glu ( 3) | Asp ( 3) | Ser ( 1) | Ala ( 1) | ||||
| Neutral | Arg ( 0) | Thr ( 0) | ||||||
| Disfavoured | Pro (-1) | Gln (-1) | Cys (-1) | Val (-1) | Lys (-1) | Asn (-2) | Ile (-2) | Trp (-2) |
| His (-3) | Met (-3) | Leu (-4) | Tyr (-5) | Phe (-5) |
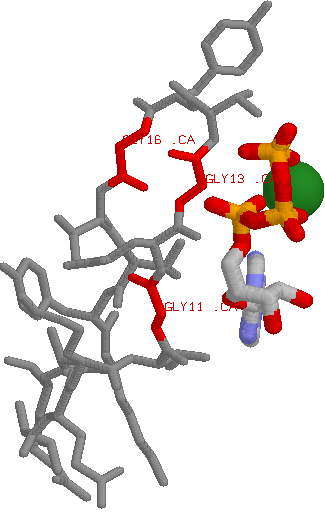
Role in structure: Glycine is a very unique amino acid in that in contains a hydrogen as its side chain (rather than a carbon as is the case in all other amino acids). This means that there is much more conformational flexibility in glycine. What this means is that glycine can reside in parts of protein structures that are forbidden to all other amino acids (e.g. tight turns in structures). Role in function: The uniqueness of Glycine also means that it can play a distinct functional role, such as using its sidechain-less backbone to bind to phosphates. This means that if one sees a conserved glycine changing to any other amino acid (i.e. even those listed above), the change could have an impact.
A good example is found in protein kinases: